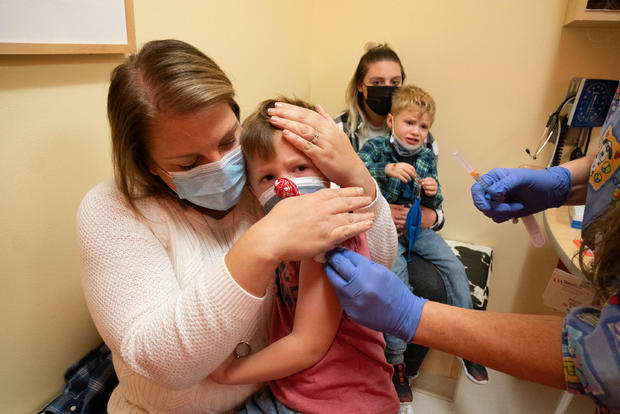
Moderna says it has formally submitted a request to the Meals and Drug Administration for authorization of two doses of its COVID-19 vaccine in kids youthful than 6 years previous, kicking off a long-awaited federal assessment course of that would quickly greenlight the primary immunizations for these youngest Individuals.
"We imagine mRNA-1273 [the Moderna vaccine] will have the ability to safely shield these kids in opposition to SARS-CoV-2, which is so vital in our continued combat in opposition to COVID-19, and might be particularly welcomed by mother and father and caregivers," Stéphane Bancel, Moderna's CEO, mentioned in a assertion.
Moderna's request is predicated on information the corporate first touted again in March finding out two 25-microgram doses of its vaccine within the age group. These doses are smaller than the two 100-microgram doses given to adults.
The corporate says that in testing the pictures amongst two teams of youngsters — 6 months previous to 23 months previous in addition to 2 years previous to below 6 years previous — they discovered a "strong neutralizing antibody response" and a "favorable security profile."
In a preliminary evaluation of lab exams collected throughout the Omicron wave, Moderna says its vaccine efficacy in opposition to an infection was 51% amongst kids youthful than 2 and 37% amongst kids from 2 to five years previous.
"These efficacy estimates are just like vaccine efficacy estimates in adults in opposition to Omicron after two doses," the corporate mentioned in an announcement.
Subsequent steps: How lengthy will it take?
Moderna's transfer marks the most important tangible step within the technique of getting vaccines prepared for younger youngsters because the FDA postponed a deliberate assembly of its exterior vaccine advisers to debate the difficulty again in February.
On the time, officers had been getting ready for the likelihood that two doses of Pfizer and BioNTech's COVID-19 vaccine formulated for the youngest youngsters may very well be rolled out by the spring.
However following a disappointing displaying within the immune response triggered by two doses of their vaccine among the many youngest kids, the businesses at the moment are ready for outcomes from three doses, which they are saying they anticipate will supply "a better degree of safety." Knowledge is anticipated from that scientific trial by this June.
Moderna additionally says it's working to broaden its personal booster shot research to guage a 3rd dose in kids as younger as 6 months previous.
As with older age teams, the FDA should now work to vet Moderna's submission earlier than granting emergency use authorization. After that, the Facilities for Illness Management and Prevention should additionally log out on up to date suggestions earlier than the pictures may be given out, below federal provide agreements governing use of the pictures.
Spokespeople for each companies say they plan to convene a gathering of their exterior vaccine advisers earlier than clearing Moderna's pictures.
The FDA's prime vaccines official, Dr. Peter Marks, instructed a Senate listening to on Tuesday that the company would launch over the following week a "tentative" schedule for his or her Vaccines and Associated Organic Merchandise Advisory Committee assembly.
Nevertheless, the corporate should first end its submission to the FDA, forking over reams of knowledge on its scientific trials and manufacturing.
"FDA can't attain a choice on any vaccine with out a accomplished EUA request, which permits us to do a radical assessment, which incorporates, amongst different issues, a complete assessment of all the opposed occasions and replication of the important thing analyses," Abby Capobianco, an FDA spokesperson, mentioned in an announcement.
Moderna mentioned Thursday that their submission "might be full subsequent week."
"We acknowledge mother and father are anxious to have their younger kids vaccinated in opposition to COVID-19 and whereas the FDA can't predict how lengthy its analysis of the info and knowledge will take, we'll assessment any EUA request we obtain as rapidly as attainable utilizing a science-based strategy," Capobianco mentioned.
All the technique of clearing Pfizer-BioNTech's pictures for youths ages 5 and up took round 27 days to finish final 12 months, from after they end submitting their request on October 6 to the CDC director signing off on the up to date suggestions on November 2.
Federal well being officers have cautioned that clearing Moderna's pictures may not occur as rapidly.
In an interview with CNN final week, requested about studies that the FDA would possibly delay its choice over Moderna's submission, the president's chief medical adviser Dr. Anthony Fauci mentioned that regulators needed to keep away from confusion between the 2 vaccines being greenlighted inside weeks of one another.
"So it may be two separate corporations, two merchandise which can be related, however not equivalent, significantly with regard to the dose. And what the FDA needs to do is to get it in order that we do not confuse folks to say that is the dose, that is the dose routine for youngsters inside that age group of 6 months to five years," Fauci mentioned.
The FDA has downplayed strategies that it's delaying the assessment of COVID-19 vaccines for the youngest kids. Requested on the Senate listening to concerning the subject, Marks hinted that Moderna's utility would possibly merely take longer for regulators to comb by way of.
"A few of these are sophisticated as a result of they're comparatively bigger, overlaying bigger swaths of the pediatric inhabitants than others," mentioned Marks.
Whereas Moderna is already cleared to be used in a number of nations for youngsters as younger as 6 years previous, solely adults are approved to obtain the 100-microgram pictures within the U.S. The corporate's submission for American teenagers has been stalled for months earlier than the FDA over issues of uncommon coronary heart irritation unwanted effects linked to the pictures.
Throughout the previous weeks, Moderna says it has additionally filed with the FDA information from its 6- to 11-year-old submission for different nations, in addition to security follow-up information in adolescents overlaying 6 months after they have been vaccinated.
The corporate's executives instructed traders earlier this 12 months that it had agreed with the FDA to review a smaller 50-microgram major sequence in adolescents, although insisting that the corporate remained assured within the "sturdy efficacy profile" of 100 micrograms outweighing the dangers in that age group.
"We care deeply concerning the well being and properly being of youngsters. So making a protected and efficient COVID-19 vaccine obtainable for youths below 5 years of age is totally certainly one of our highest priorities. However merely making a vaccine obtainable does not matter if mother and father are hesitant to get their youngsters vaccinated," Marks mentioned in a video posted by the FDA on Tuesday.
Solely round 28% of youngsters ages 5 to 11 years previous are totally vaccinated and vaccine hesitancy amongst mother and father of this age group has climbed in current months. Near 4 in 10 mother and father of youngsters within the age group say they in all probability or undoubtedly is not going to get their youngsters vaccinated, as of the CDC's final immunization survey.
"However let me be very clear, being thorough completely doesn't imply we're delaying assessment of those vaccines. We're going to transfer with all expediency, with out sacrificing our requirements, to finish our evaluations," Marks added later.